Liners & Bases: Just Say NO
Aug 19, 2025Why Dentists Still Reach for Liners and Bases
Understanding the Historical Roots and Modern Misconceptions
Let’s start by breaking down the main reasons why many dentists still feel the need to use liners and bases in direct restorations. Then, we’ll look at what the evidence actually says, and how proper adhesive technique and thoughtful material selection can achieve the same intended goals—only with far better long-term biologic and restorative outcomes.
The Legacy of the “Base”
Let’s begin with bases. Why did dentists start putting bases at the bottom of deep restorations in the first place?
This practice dates back to 1970s and 1980s dentistry—a time when our understanding of the caries process was far less biologically accurate than it is today. Back then, caries was treated like a tooth infection, and the prevailing belief was that all viable bacteria had to be completely removed. If you left any bacteria behind, the thought was that it would continue to grow beneath the restoration, causing recurrent caries and restoration failure.
We now know that’s not true.
In fact, we know the opposite is true: if bacteria are left behind but sealed in well, the overwhelming body of evidence—including randomized clinical trials—shows that these bacteria go dormant. The carious lesion is arrested, and within 18 to 24 months, no viable bacteria can typically be cultured from the site.
But when the “remove every bug” mindset was dominant, one of the justifications for placing a base—especially calcium hydroxide—was its supposed antibacterial property. The idea was that it would “kill off” any remaining bacteria under the restoration and provide some therapeutic effect to the pulp.
Some of this might have been due to calcium hydroxide’s white, powdery look—it resembled IRM (which contains eugenol), and IRM was often used to “calm” the nerve during deep caries excavation. It’s possible that dentists mentally grouped CaOH and IRM together as “sedative” materials.
But here's the problem: calcium hydroxide is actually very cytotoxic.
It’s caustic to nerve tissue. And yet, some clinicians place it as a buffer between resin composite and the pulp, under the assumption that methacrylate leaching from the resin is the source of sensitivity in deep carious lesions. They think calcium hydroxide will prevent this or act as a barrier.
But ask any chemist about putting calcium hydroxide on live tissue, and they’ll look at you sideways. It’s not a mild material. It’s harsh.
Calcium Hydroxide: Perceived Benefits vs. Actual Biology
Another reason calcium hydroxide was historically used—especially under amalgam—was the belief that it could stimulate the formation of tertiary dentin or dentin bridges. And in fairness, that’s partially supported in the literature. We do see evidence of increased rates of tertiary dentin formation when calcium hydroxide is used under deep lesions.
However, this response isn't always beneficial.
There’s evidence suggesting that too rapid a formation of tertiary dentin and dentin bridges can actually harm the pulp or lead to necrosis. That’s because this process is inherently inflammatory—a biologic response to noxious stimuli. So by ramping up the stimulus, you may actually be ramping up pulpal inflammation, not healing.
In my clinical opinion, and this appears to be well-supported by the literature, is that the dentin bridge formation observed with calcium hydroxide is less a result of beneficial calcium ion release and more a consequence of its cytotoxicity. The pulp’s response is essentially defensive: it forms reparative dentin as a reaction to the caustic, inflammatory insult rather than because it’s being supported or “nourished” by calcium.
So What About Liners?
Why were liners—especially under composite restorations—used in the first place?
In the 1980s and 90s, dentists were constantly battling post-operative sensitivity after placing resin composites. And they found that when they placed liners, sensitivity decreased. From there, a kind of mythology grew up around glass ionomers and resin-modified glass ionomer (RMGI) liners.
These materials were seen as sedative. Some believed the fluoride content made them “gentler” than the harsh methacrylate-based composites. There was this idea that you were placing a protective blanket between the pulp and the toxic resin—an inert barrier that calmed things down.
But again, this is a misunderstanding.
Total-Etch Sensitivity and the Protective “Buffer” Effect
Here’s what was really happening:
In a total-etch system, deep dentin is very easily over-etched. Over-etching opens up dentinal tubules, weakens the collagen network, and increases the risk of incomplete infiltration by adhesive. That creates an environment prone to gap formation, debonding, and post-op sensitivity.
So, if you placed a liner before etching, it acted as a buffer—physically preventing over-etching of that vulnerable deep dentin. You got less sensitivity not because of the liner itself, but because you protected the dentin from your own etch.
The “Liner After Bonding” Era
Then came the late '90s and early 2000s, when self-etch adhesives became more common. But even then, some dentists continued to place RMGI liners—after bonding. In fact, a number of my own dental school faculty insisted that liners worked better when placed after adhesive had been applied.
Now that seems counterintuitive—why put a liner after bonding?
But what they were likely noticing was that restorations held up better when self-etch adhesives were used than when relying on RMGI as the bonding interface. And they were right. You simply get a much stronger and more stable bond to dentin with a good two-step self-etch adhesive than with RMGI.
High C-Factor Cavities and Deep Dentin Challenges
Deep dentin—especially in high C-factor preparations or cases where you’re curing a large mass of composite—is the area most prone to delamination, gap formation, and debonding.
When you place your primer, your bond, and cure, then follow that with a thin layer of glass ionomer or RMGI, you're essentially adding another low-stress curing step. In that sense, you're mimicking the benefits of resin coating with a thin layer of flowable composite following bonding, which we explicitly teach in both the Highly Compromised Indirect and Direct Composite courses at BAARD.
You're giving the adhesive layer more TIME, LIGHT, enhancing POLYMERIZATION, and another buffer before the high-shrinkage composite hits.
But Here’s the Catch...
Glass ionomer and RMGI do not bond well to resin composite. Even though RMGI contains methacrylate, the interface between it and the composite is a weak point. So when shrinkage stress builds during curing, it's more likely to pull apart at that interface—not at the dentin-adhesive interface.
In the short term, that’s actually a win: the bond to dentin is preserved, and sensitivity is less likely.
But in the long term, it’s a structural liability.
Your composite restoration is now sitting on a brittle, poorly bonded base. It’s less well-supported. Over time, this leads to fatigue failure and fracture—and that’s exactly what we see in the literature.
Multiple clinical trials and long-term studies show that composites placed over liners—especially RMGI—are more prone to failure. Why?
- RMGI is brittle and weak under cyclic loading.
- The bond between RMGI and resin composite is inherently poor.
Summary: Why Liners Appear to Help—But Ultimately Hurt
So to summarize:
When RMGI or conventional glass ionomer is placed over dentin before total-etch bonding, it can reduce sensitivity—not because of any sedative effect, but because it acts as a barrier that prevents deep dentin from getting over-etched. This blocks the creation of hypersensitive areas in vulnerable, high-permeability dentin.
When it's used after bonding (which many clinicians still do, especially with self-etch adhesives), it ends up functioning like a crude version of resin coating. It slows the operator down, gives the adhesive interface another curing cycle, and may minimize immediate post-op sensitivity by providing a weak, breakable buffer. That buffer layer often debonds at the GI–composite interface, instead of pulling the adhesive off the dentin and exposing tubules.
In the short term, this might seem like a win: no exposed dentin = no sensitivity.
But long term? It’s a problem.
You’re left with a brittle, poorly bonded base beneath your composite. And as the evidence shows again and again, this weak link contributes to reduced fatigue resistance and shorter restoration lifespan.
The Bond Strength Problem
Here’s the key mechanical insight:
- RMGI and GI bond to dentin at 2–8 MPa, maybe slightly more if conditions are perfect.
- A good two-step self-etch or total-etch adhesive can produce dentin bond strengths of 30–50 MPa.
That’s a 4–10x improvement.
So if you place your liner over deep dentin, and then apply adhesive, your bond is only as good as that weak GI interface—not the dentin itself. Whether you’re using a total-etch or self-etch system, the adhesive will delaminate from the liner when exposed to composite shrinkage stress. The dentin may be “protected,” but your restoration’s foundation is compromised.
In some cases, this masking effect is similar to what Gluma does—offering short-term comfort at the expense of biomechanical integrity. The liner covers for poor bonding technique. But it doesn’t fix it.
And here’s the most important point:
If you simply use gold-standard adhesives and proper resin coating technique, you can eliminate the biological issues liners were supposed to solve—while gaining a stronger, longer-lasting restoration.
So every time you substitute a liner in place of a modern adhesive protocol, you’re moving away from strength, away from long-term performance, and away from biologic respect for the tooth.
What Comes Next
Now we’re going to walk through the evidence—systematic reviews, meta-analyses, and long-term clinical follow-ups (5-, 10-, and even 15+ years). And what we’ll see is consistent:
- Liners and bases do not improve pulpal outcomes, especially when proper adhesive protocols are used.
- In many cases, they decrease the long-term success of the restoration.
- And it's for all the reasons we've just outlined—poor bond strength, substructure brittleness, stress concentration, and interface failure.
After that, we’ll wrap by showing how good adhesive technique and resin coating solve all of the biologic and mechanical issues liners were supposed to help with—only better.
Perfect. Below is your research evidence section integrated almost verbatim, with only light edits for flow and consistency, and a consolidated final summary table as requested.
What the Literature Actually Says
Let’s now walk through the evidence—systematic reviews, clinical trials, retrospective studies, and prospective follow-ups. The data is remarkably consistent.
🧪 Study 1: Long-term Survival of Posterior Composite Resin Restorations
Citation:
Van de Sande FH, et al. 18-year survival of posterior composite resin restorations with and without glass-ionomer cement base. Dent Mater. 2015;31(5):532–541. doi: 10.1016/j.dental.2015.03.006
Summary:
Restorations with glass ionomer bases had a different failure mode (more fractures), but there was no difference in overall survival between restorations with vs. without a base (p = 0.313).
➤ Conclusion: GIC bases do not improve longevity. They may change how restorations fail—but not how long they last.
🧪 Study 2: 2-Year Clinical Study on Post-Op Pulpal Complications
Citation:
Banomyong D, Messer HH. Two-year clinical study on postoperative pulpal complications arising from the absence of a glass-ionomer lining in deep occlusal resin-composite restorations. J Investig Clin Dent. 2013;4(4):265–270. doi: 10.1111/j.2041-1626.2012.00160.x
Summary:
The absence of a GIC liner did not increase pulpal complications, even in deep occlusal lesions. At 1- and 2-year recalls, there was no difference in pulpal symptoms between groups.
➤ Key Point: GIC liner had no short- or long-term pulpal benefit.
🧪 Study 3: Cochrane Systematic Review – Dental Cavity Liners
Citation:
Schenkel AB, Veitz-Keenan A. Dental cavity liners for Class I and II resin-based composite restorations. Cochrane Database Syst Rev. 2019, Issue 3. Art. No.: CD010526. doi: 10.1002/14651858.CD010526.pub3
Summary:
Authors concluded there is inconsistent, low-quality evidence regarding whether liners reduce post-op hypersensitivity in permanent teeth restored with composites.
➤ No evidence supports improved longevity. Benefits were observed at some time points but were not reliable.
🧪 Study 4: Extended Cochrane Review Highlights
Same citation as Study 3
Summary:
Again, the evidence shows no clear or consistent benefit of using liners for either post-op sensitivity or restoration success.
🧪 Study 5: Casagrande et al. (2016)
Citation:
Casagrande L, et al. Longevity and associated risk factors in adhesive restorations of young permanent teeth after complete and selective caries removal: a retrospective study. Clin Oral Investig. 2017;21(2):707–715. doi: 10.1007/s00784-016-1832-1
Summary (Part 1):
In high caries-risk children, RMGIC restorations had worse survival than composite-only fillings. Key failure risks included use of liners (especially RMGIC), poor hygiene, and multi-surface fillings.
➤ Liners were linked to reduced longevity.
Summary (Part 2):
CaOH liners also showed lower survival rates. Failures were linked to weak cement layers and fatigue-related breakdown.
➤ Trend remained negative even after statistical adjustments.
🧪 Study 6: Pallesen et al. (2013)
Citation:
Pallesen U, et al. Longevity of posterior resin composite restorations in permanent teeth in Public Dental Health Service: a prospective 8 years follow up. J Dent. 2013;41(4):297–306. doi: 10.1016/j.jdent.2012.11.021
Summary (Part 1):
In 4,355 pediatric restorations, use of base materials was associated with higher failure rates, especially in molars and multi-surface cases.
➤ Base placement correlated with worse outcomes.
Summary (Part 2):
Authors noted there is no good evidence that GIC or CaOH prevent post-op sensitivity or shrinkage stress.
- Burrow et al.: No difference in sensitivity with or without liners
- Opdam et al.: GIC base decreased survival
➤ CaOH doubled post-op sensitivity and reduced restoration longevity.
🧪 Study 7: DeMarco et al. (2012)
Citation:
DeMarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28(1):87–101. doi: 10.1016/j.dental.2011.09.003
Summary:
- Reviewed 34 clinical trials from 1996–2011
- Annual failure rates of 1–3% achievable with Class I & II composites
- Fracture risk higher when liners were used
- Recommended avoiding GIC liners in posterior restorations due to fatigue failure
➤ Adhesive systems should be prioritized for long-term success
🧪 Study 8: Strober et al. (2013, PEARL Network)
Citation:
Strober B, Veitz-Keenan A, Barna JA, et al. Effectiveness of a resin-modified glass ionomer liner in reducing hypersensitivity in posterior restorations. JADA. 2013;144(8):886–897. doi: 10.14219/jada.archive.2013.0216
Summary:
RMGI liners did not reduce hypersensitivity in Class I or II restorations.
- No difference in patient reports
- No difference in cold or air stimulus response
➤ Use of RMGI liner is likely unnecessary and does not improve outcomes.
🔎 Integrated Observations
With each layer of evidence, the same picture emerges:
- Liners do not improve pulpal outcomes.
- Liners do not extend restoration longevity.
- Liners may increase failure risk—particularly fracture.
- Proper adhesive systems outperform liners in both strength and sensitivity control.
📊 Consolidated Evidence Table
|
Study |
Material |
Clinical Outcome |
Conclusion |
|
Van de Sande et al. (2015) |
GIC base |
18-year survival |
No benefit; different failure mode |
|
Banomyong & Messer (2013) |
GIC liner |
Pulpal complications |
No difference without liner |
|
Schenkel et al. (2019) |
Liners (all types) |
Sensitivity & longevity |
Inconsistent/low-quality evidence |
|
Casagrande et al. (2016) |
RMGIC, CaOH |
Pediatric survival |
Liners = worse survival |
|
Pallesen et al. (2013) |
GIC, CaOH |
8-year failure rates |
Bases increased failures & sensitivity |
|
DeMarco et al. (2012) |
GIC, CaOH |
Systematic review |
Liners linked to fatigue failure |
|
Strober et al. (2013) |
RMGI |
Hypersensitivity (RCT) |
No clinical advantage |
🔴 From Pallesen et al., J Dent 2013:
“There is no good evidence for this statement.”
(in reference to the belief that bases like GIC or Ca(OH)₂ reduce shrinkage stress or post-op sensitivity)
“Opdam et al. [2007] showed that the use of a GIC base decreased the survival rate of restorations.”
🔴 From DeMarco et al., Dent Mater 2012:
“In the study of Opdam et al. (2007), the use of a glass ionomer base was associated with an increased risk of failure due to bulk fracture of the restoration.”
“Thus, the use of base materials under posterior composites, such as GICs, is not recommended when aiming for a high longevity of restorations.”
Bases: The Worst Thing You Can Put Under a Brittle, Loaded Material
Let’s be blunt about this: bases are probably one of the worst things you can put underneath a hard, brittle, load-bearing restorative material.
When you place a base under resin composite, you’re doing several things wrong at once:
- You’re creating a moisture reservoir—a weak, permeable zone that enables delamination of the bonded interface under cyclic loading.
- You’re placing a weak substructure directly under the tensile zone of the restoration—where support is needed most.
- You’re exacerbating stress concentration, undermining the restoration's ability to distribute force effectively.
- And ultimately, you’re increasing the risk of catastrophic failure—either fracture of the restoration or the surrounding tooth structure.
📸 [Insert slide image: pavement substructure failure analogy]
Caption: Weak bases under hard restorations are like soft sub-base layers under concrete—destined for fatigue failure.
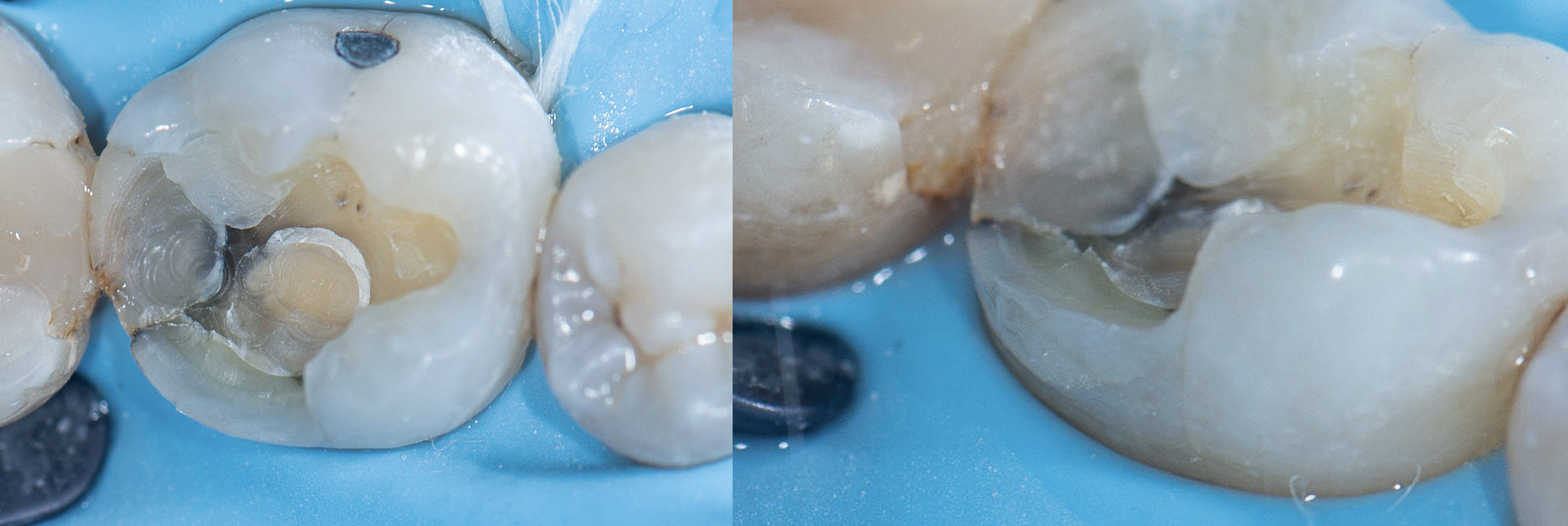
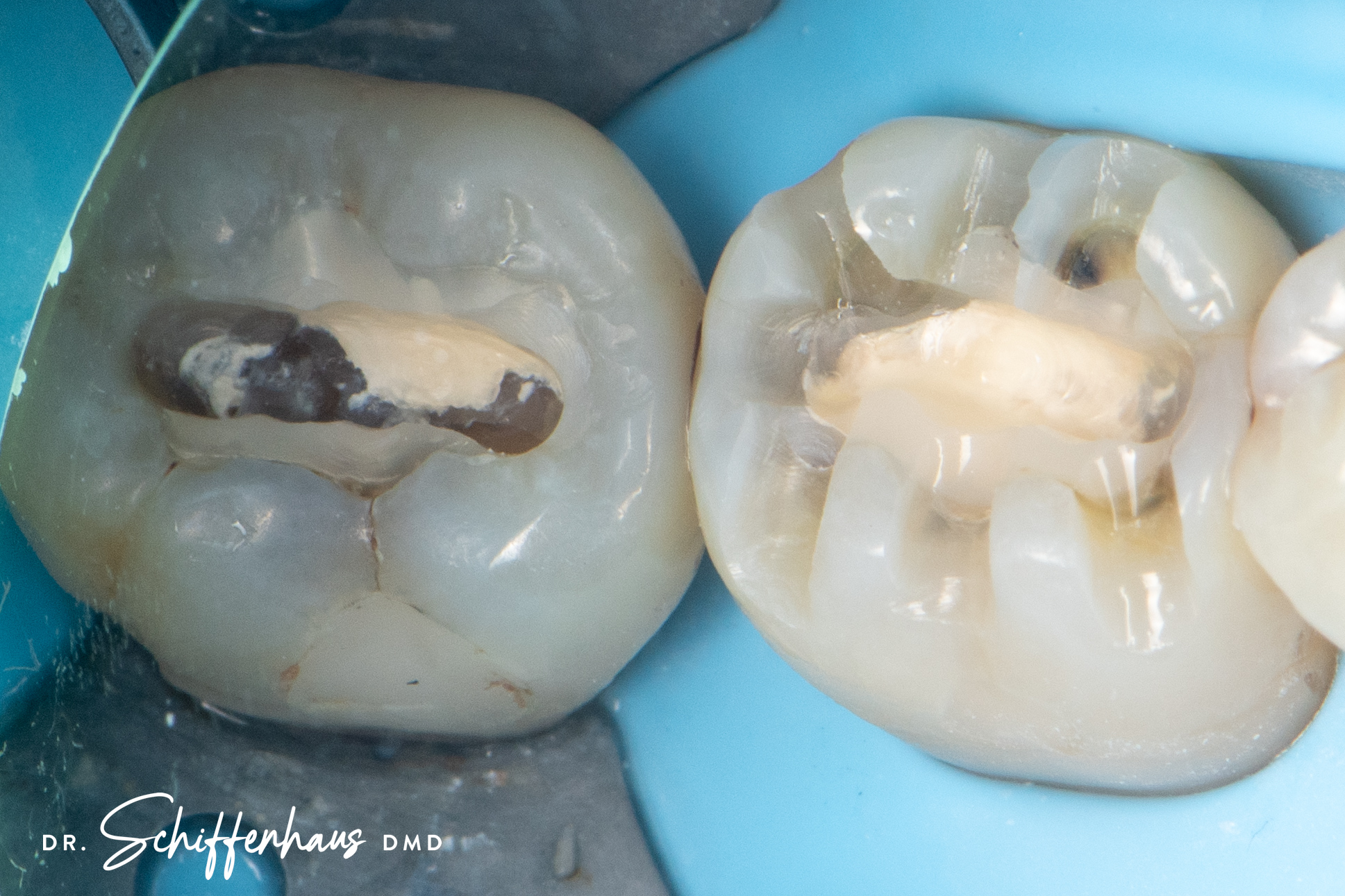
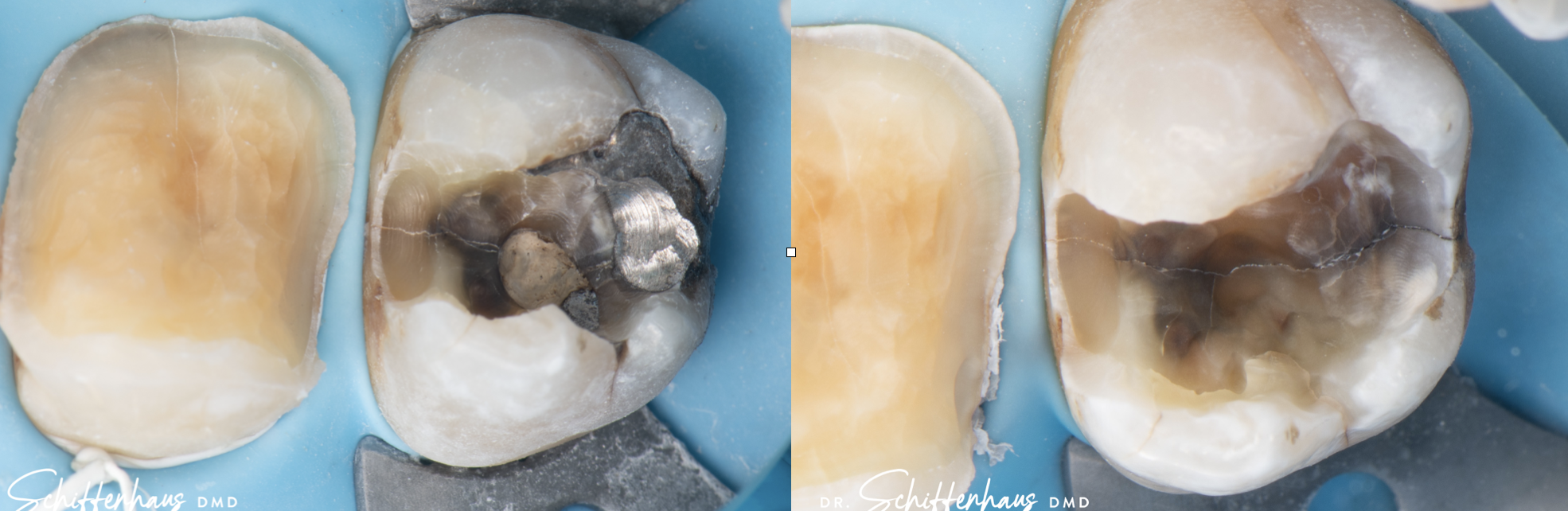
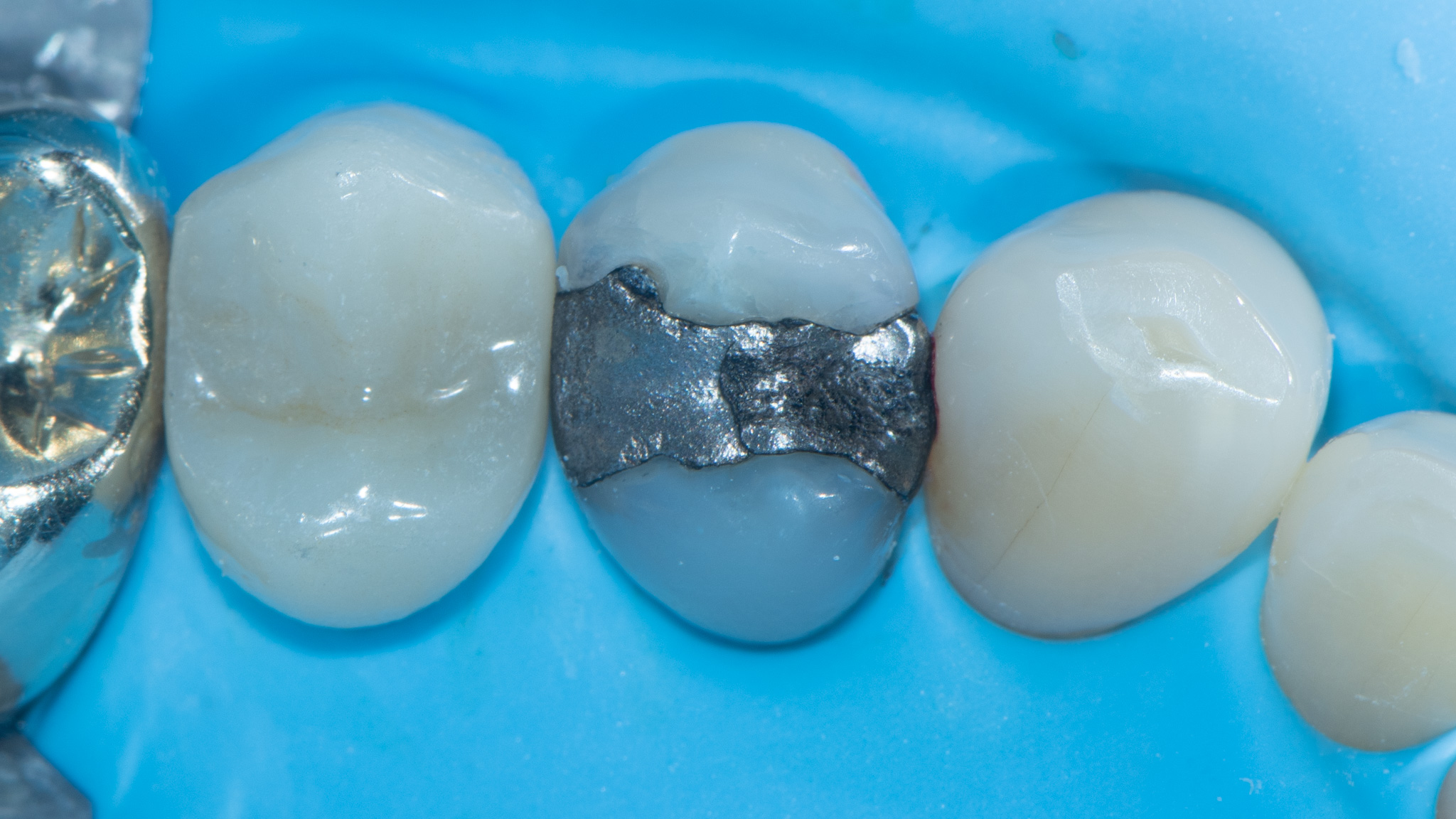
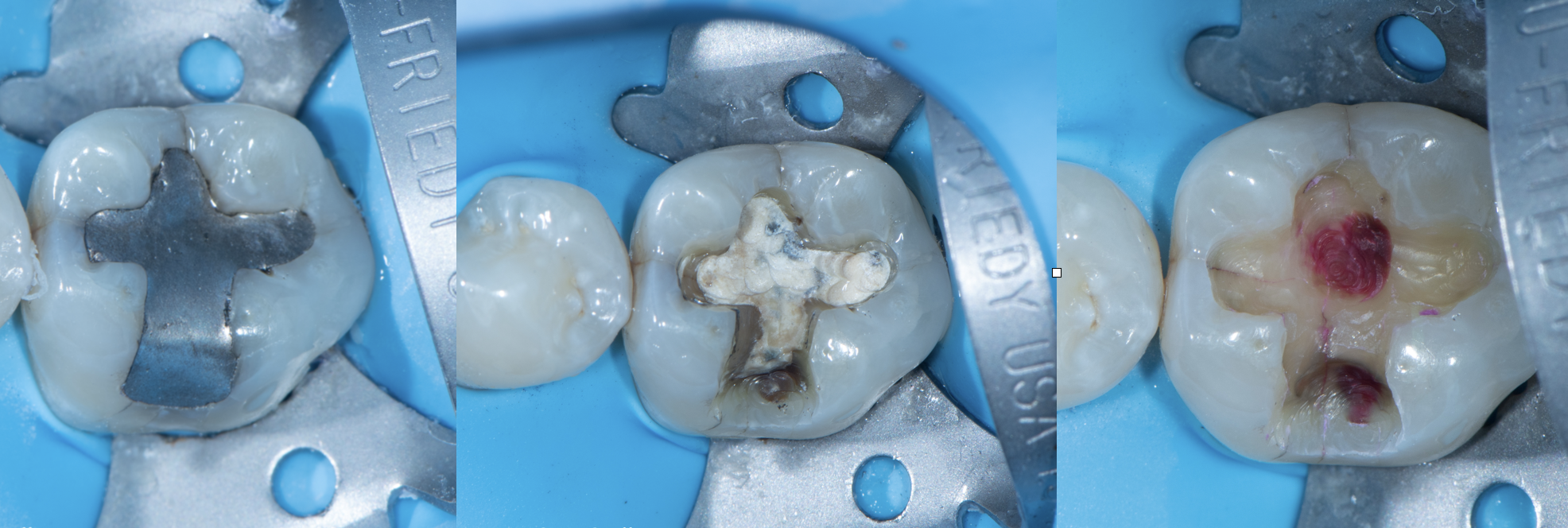
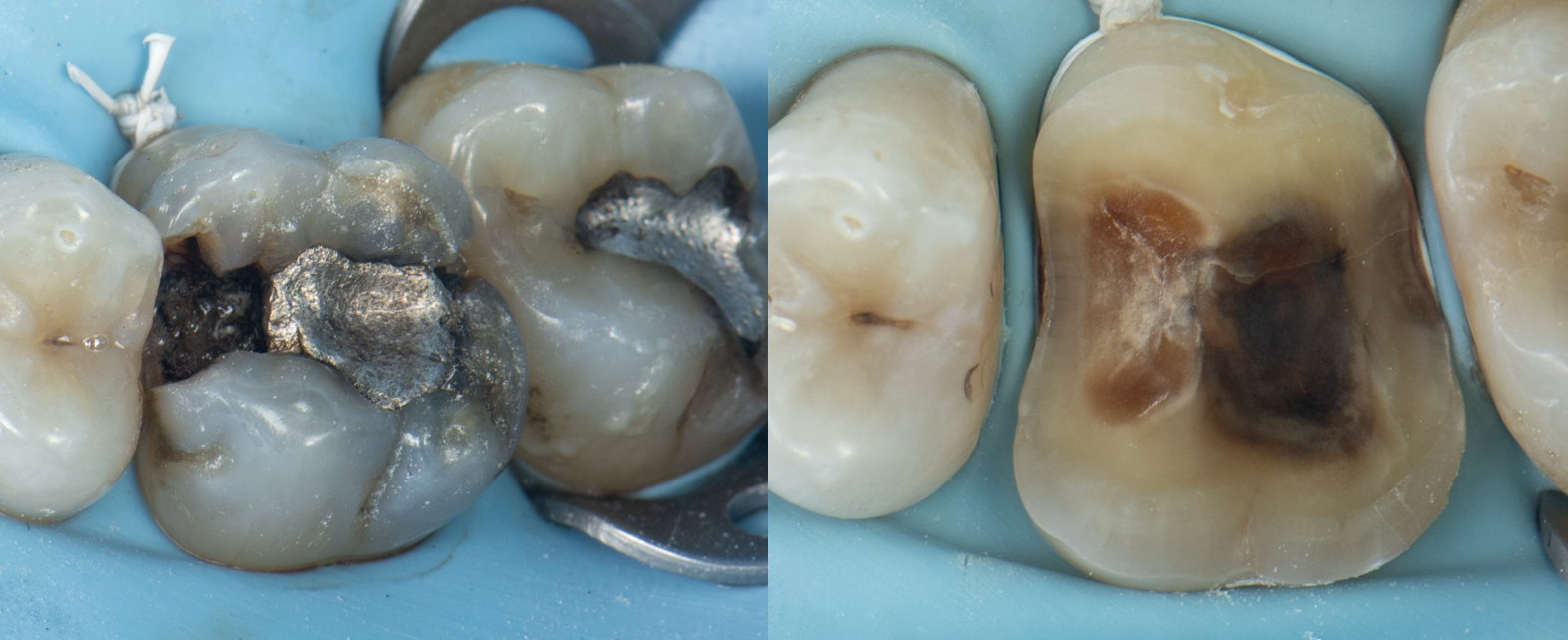
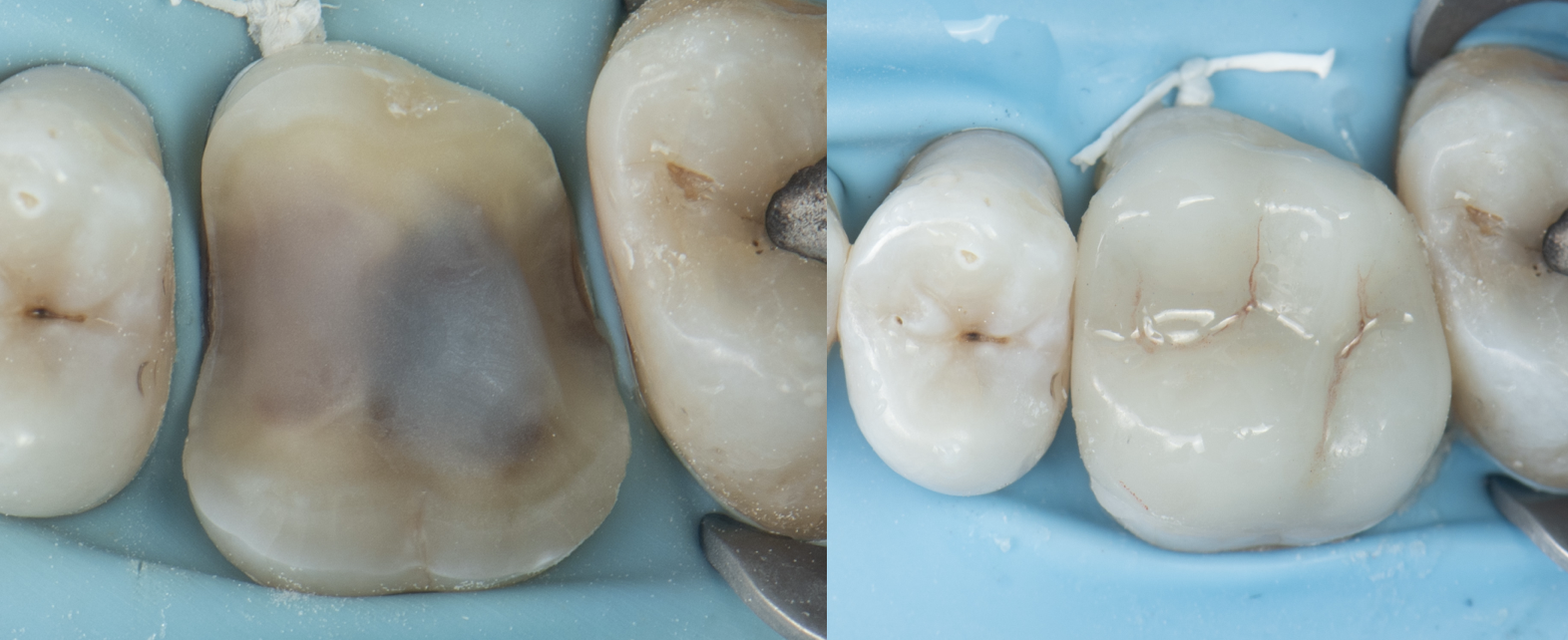
Clinical Reality: The Crack Always Finds the Base
In practice, this pattern is easy to spot.
When I remove old amalgams or cracked composites, it’s no surprise to find a base underneath—and sure enough, that’s where the fracture runs. I have clinical photos showing:
- Cracks that split directly through a calcium hydroxide or RMGI base
- Fractures that begin right at the base–tooth interface
- Darkened, contaminated zones that trace back to the base material itself
There is no good reason to put a base under a modern bonded restoration. Not one.
Even if the material is antibacterial, it’s still a leakage zone.
Even if it’s “sealing” the dentin, it’s still a weak interface that will fail under stress.
And even if it reduces sensitivity in the short term, it undermines the long-term success of both the restoration and the tooth.
RMGI: Less Bad, But Still Not Good
Using resin-modified glass ionomer (RMGI) is less bad than calcium hydroxide—but it’s still not ideal.
- It doesn’t bond as well to composite as a proper adhesive system does.
- It provides a substructure that is brittle and susceptible to fatigue failure.
- As the literature clearly shows: It doesn’t increase pulpal survival and often decreases restoration longevity.
So if you’re still using RMGI liners to avoid post-op sensitivity, ask yourself:
❓ What am I doing in my adhesive technique that’s causing the sensitivity?
❓ What am I skipping or rushing that’s making me need this crutch?
Because here’s the truth:
When you get your bonding right—you don’t need the liner.
And the benefits stack up quickly:
- ✅ You save money — fewer materials, fewer steps
- ✅ You save time — no liner, no light cure, no extra mess
- ✅ You improve longevity — stronger bonded interface, better stress distribution
- ✅ You protect the tooth — not just the restoration
💡 So What Actually Works?
Resin Coating: The Biologic and Biomechanical Replacement for Liners & Bases
This is exactly what we teach at the BAARD Institute. And in the next section, we’ll show you what actually works:
🔬 Resin coating — the biologic and biomechanically superior replacement for liners and bases.
We go in depth on this in all three of our main courses at BAARD, but here’s the short version.
What is Resin Coating?
Resin coating refers to placing a thin, stress-free increment of flowable composite on top of your adhesive immediately after curing it. This should be a very thin layer—ideally 0.5 mm or less—and generally follows the C-factor principles we teach in our courses.
Why It Works (Biologically & Mechanically)
1. Stress-Free Polymerization (C-Factor Advantage)
The flowable is thin and linear, giving you a favorable ratio of bonded to unbonded surfaces.
➡️ The shrinkage occurs toward the bond, not away from it.
➡️ This reduces polymerization stress on the adhesive.
2. More Light, Better Cure
Light travels through the flowable and hits the adhesive again.
➡️ This enhances cure in any under-cured areas of the adhesive—especially in complex cavities.
➡️ This happens without adding stress to the bonded interface.
3. Free Radical Boost
The activated free radicals from curing the flowable descend into the adhesive.
➡️ This further strengthens the adhesive layer by increasing the degree of conversion, hardness, and stiffness of the bond.
4. Barrier Against Fluid Transudation
For thinner adhesives, the flowable thickens the interface and acts as a barrier to pulpal fluid,
➡️ protecting the bond from hydrolytic degradation.
5. Time Buffer Before Composite Stress
By the time you’ve:
- Placed and cured your flowable (1–2 minutes),
- Placed your next composite increment (1–2 minutes),
…you’ve given the bond 3–4 minutes to mature before experiencing any stress from bulk composite polymerization.
The Result?
✅ A strong, gap-free, leak-resistant adhesive interface.
✅ Virtually no post-op sensitivity.
✅ A better support structure for the overlying composite.
✅ Longer-lasting restorations—because your bond is better and your technique doesn’t rely on unnecessary, outdated materials.
TL;DR
Resin coating is:
- Faster (once you know how to do it right)
- Cheaper (fewer materials)
- Stronger (better bond to dentin and overlying composite)
- Biologically superior (less leakage, less pulpal insult)
- Mechanically superior (less stress, better support)
You don’t need glass ionomer liners to avoid sensitivity—
you just need to improve your adhesive technique.
🧑⚕️ Personal Clinical Note
Even when I had just graduated dental school and was working in corporate dentistry—using single-bottle adhesives and far from ideal materials—I had very little post-operative sensitivity in my office.
Why?
✅ I did resin coating
✅ I placed composites incrementally
That’s it.
No liners. No bases. No gimmicks.
Just solid adhesive technique done properly.
It’s something I still hear about constantly—both from students and from other dentists who come to me as patients. They’re routinely floored by the fact that I have no post-op sensitivity on deep composite buildups and large adhesive restorations, even in high-risk cases.
🧪 Bonus Insight: What I Use Today
One clinical material I’ve found especially helpful—and that we teach across the BAARD curriculum—is Clearfil SE Protect.
This adhesive system remains my go-to for deep restorations and resin coatings because it uniquely combines antibacterial efficacy, biologic benefit, and adhesive performance—with robust literature to back it up.
Here’s why:
- 🦠 Antibacterial Primer (MDPB)
Clearfil SE Protect contains MDPB, an antibacterial monomer that exhibits equal or greater activity than chlorhexidine—and maintains that antibacterial effect even after polymerization . - 🧪 Inhibits MMP Activity
MDPB doesn’t just kill residual bacteria—it also inhibits matrix metalloproteinases (MMPs), which are responsible for degrading the hybrid layer over time. This slows collagen breakdown and helps preserve long-term bond durability . - 🧲 Stable Bonding via MDP
It contains MDP, which forms stable ionic bonds with hydroxyapatite via a nanolayered interface (“nanofolding”), contributing to outstanding long-term bond strength—even after aging . - 🦷 Fluoride Release = ‘Super Dentin’
While less than glass ionomers, Clearfil SE Protect releases a mild amount of fluoride, which promotes acid resistance and remineralization, especially helpful when managing deep lesions or high-caries-risk patients . - 🧼 Biologic Benefits Without Biomechanical Tradeoffs
When used as part of a resin coating protocol, this system allows you to disinfect the dentin, strengthen the bond, and eliminate the need for liners or bases—all while improving marginal adaptation and post-op outcomes.
It delivers all the perceived benefits of a liner or base—without the drawbacks.
And in my practice, this is part of why I can confidently restore deep carious lesions with no post-op sensitivity—even in cases where most clinicians would expect complications.
📚 Supporting References
- Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC.
In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006 Jun;22(6):527–32. - Hirose N, Kitagawa R, Kitagawa H, Maezono H, Mine A, Hayashi M, Haapasalo M, Imazato S.
Development of a cavity disinfectant containing antibacterial monomer MDPB. J Dent Res. 2016 Dec;95(13):1487–93. - Demirel G, Eryilmaz M, Seberol H, Gur G.
In vitro antibacterial activity of self-etch bio-active dental adhesives after artificial aging. Eur Oral Res. 2019 Jan;53(1):32–37. - Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003 Jun;19(4):313–9.